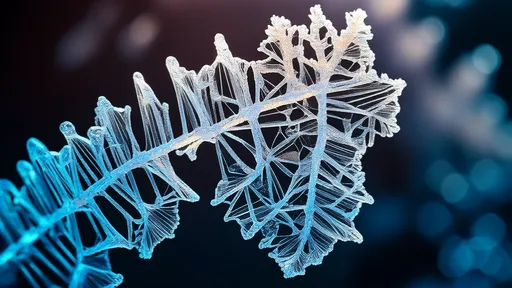
The delicate dance between science and art finds one of its most enchanting expressions in the crystalline branching structures of sugar formations. Often referred to as "rock candy trees" or "sugar fractals," these intricate edible sculptures blur the boundaries between molecular chemistry and natural aesthetics. What begins as a simple supersaturated sugar solution transforms, through patient evaporation, into breathtaking dendritic architectures that mirror the patterns of frost on windows or branches against winter skies.
The magic begins at the molecular level, where sucrose molecules obey the same physical laws that govern snowflakes and mineral deposits. When a sugar solution reaches supersaturation – that precise moment when water can no longer hold all the dissolved sugar – molecules begin their exquisite self-assembly. Unlike the random clumping one might expect, the crystallization process follows remarkably organized patterns, building upon minute imperfections and irregularities in the container's surface to establish its growth points.
Temperature plays the role of invisible choreographer in this crystalline ballet. A gradual cooling process encourages the formation of larger, more defined crystals, while rapid temperature changes produce finer, more delicate structures. The viscosity of the solution determines how easily sugar molecules can travel to join the growing crystal lattice, affecting whether the final structure appears feathery or chunky. These variables combine to create unique formations every time, ensuring no two sugar fractal trees are perfectly identical.
What fascinates scientists and artists alike is the uncanny resemblance these edible crystals bear to natural fractal patterns. The branching angles of sugar formations often approximate 60 degrees, mirroring the geometry seen in ice crystals and certain mineral deposits. This phenomenon occurs because sucrose molecules arrange themselves in a hexagonal lattice when crystallizing, creating the foundation for these mathematically perfect angles. The recursive branching pattern – where smaller branches mimic the structure of larger ones – demonstrates how simple rules at the microscopic scale can produce astonishing complexity visible to the naked eye.
The process of growing these crystalline trees has become both a scientific demonstration and a meditative art form. Enthusiasts carefully prepare their sugar solutions, sometimes adding food coloring or flavorings to enhance the visual and gustatory appeal. The choice of surface for crystal nucleation – whether a rough string, a wooden stick, or even another sugar crystal – influences the final form. Some practitioners manipulate the environment by adjusting humidity levels or creating gentle air currents to guide the growth direction, resulting in asymmetrical structures that appear to bend toward imagined sunlight.
Beyond their beauty, sugar fractals serve as edible demonstrations of fundamental scientific principles. They illustrate diffusion-limited aggregation, where particles moving randomly through a solution become permanently attached to a growing structure. The branching patterns emerge from competition between sugar molecules attempting to join the crystal from different directions – a microscopic version of plants competing for sunlight in a dense forest. This makes sugar crystal growth an excellent hands-on experiment for introducing complex concepts like supersaturation, nucleation, and crystal lattice formation to students of all ages.
The intersection of culinary art and materials science becomes particularly intriguing when examining how impurities affect sugar crystallization. Small amounts of other substances – whether intentionally added flavors or accidental contaminants – can dramatically alter the crystal morphology. Cream of tartar, for instance, promotes the formation of numerous small crystals rather than fewer large ones, creating structures with a distinctly different visual texture. This sensitivity to environmental conditions makes sugar crystallization a surprisingly sophisticated process, one that challenges the distinction between kitchen chemistry and laboratory science.
Modern technology has brought new dimensions to this ancient phenomenon. Time-lapse photography reveals the mesmerizing growth patterns of sugar crystals in ways the naked eye could never perceive. Computational models can simulate crystal growth under various conditions, helping researchers understand not just sugar crystallization but similar processes in metal alloys and geological formations. Some contemporary artists have even incorporated electrically conductive sugar crystal formations into interactive installations, where the delicate branches complete circuits to trigger lights or sounds when touched.
As both a scientific curiosity and an artistic medium, sugar fractal trees continue to captivate. They remind us that nature's patterns repeat across scales, from microscopic molecular arrangements to the branching of galactic arms. The next time you stir sugar into your tea or admire the frost on your window, consider the invisible forces shaping those crystals – and perhaps be inspired to grow your own edible fractal forest, one molecule at a time.

By /Jul 7, 2025

By /Jul 7, 2025

By /Jul 7, 2025

By /Jul 7, 2025
By /Jul 7, 2025

By /Jul 7, 2025

By /Jul 7, 2025

By /Jul 7, 2025

By /Jul 7, 2025

By /Jul 7, 2025

By /Jul 7, 2025

By /Jul 7, 2025

By /Jul 7, 2025

By /Jul 7, 2025

By /Jul 7, 2025

By /Jul 7, 2025

By /Jul 7, 2025
By /Jul 7, 2025